Pharmaceutical Label Inspection Drastically Reduces Medical Study Prep
LabelPack Automation
Oct 16, 2025 4:14:30 PM

The problem – Production times were inefficient due to manual label inspections
When representatives of one of the largest pharmaceutical companies approached LabelPack Automation, they had several concerns with their current labeling and packaging process. Their main concern was the amount of time it took for a product to be packaged, labeling to be approved, labeling to be applied, and the item to be shipped to their testing facilities. The process took an average of 3 months for a 20,000 piece study – a much longer time than the average in the drug industry. Their second concern involved the printing of the labels used in blind studies. Pharmaceutical labeling guidelines require all medicinal ingredients to be labeled on the bottle or package in both sales and medical testing. With a blind test, the pharmaceutical ingredients must still be labeled according to drug safety requirements, but the medical testing participants cannot be aware of the properties of the drug unless there is a medical emergency. For this, the client was in need of a separate label application system and label stock designed for blind testing. After talking in length about the pharma company's print-and-apply concerns, LabelPack Automation began designing the two different labeling systems to solve the client's application requirements.Phase One: Pharmaceutical Label Inspection System
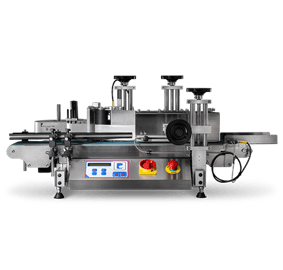
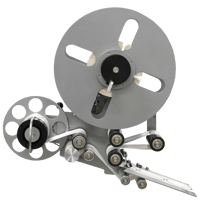
To increase throughput for this pharma company, LabelPack Automation designed a custom Pharmaceutical Label Inspection System. The labeling system supplies labels to a thermal printer, which transfers those labels through an inspection station with a vision system, and winds them onto a roll or advances them to a fanfold tray. Instead of hand-inspecting every label as they are printed – the company's previous mode of inspection – this system gives you the option of manual-inspection, automatic inspection (vision system), or both. The Pharmaceutical Label Inspection System has a loose loop control that activates the printer as labels are fed through the system in Single Panel Mode. If there are no labels detected in the loop, the system does not index until labels are printed. While the printer is in Booklet mode, the Camera – also called the Opto-touch Switch or the optional Foot Switch – activates the printer. The system developed for this pharma company can be used in manual or automatic modes. While in manual mode, the operator uses the Opto-Touch Switch or the optional Foot Switch to begin the feed-cycle. A thru beam sensor detects the label stock and continues to feed to a pre-set distance. Once the label is indexed into position, it is inspected by the operator before the next label is fed through. If the system is in automatic mode, the operator depresses the cycle start button to begin the process. A thru beam sensor detects the label and continues to feed the label to the pre-set distance. The vision system scans the label and provides either a “good read” signal or a “bad read” signal to the feeding system. If the inspection is completed by the vision system, the camera must give a “good read” signal before the next label is fed through to ensure quality labels every time. However, if the vision system signals a “bad read,” the entire labeling system defaults into manual mode. The operator will then have to place the system back into auto and depress the cycle button before the next cycle begins.Phase Two: Blind Test Labels
The next phase of the two part solution LabelPack Automation has planned for the pharma company is designed to solve their need for labels that comply with drug safety guidelines and allow them to complete blind testing in their facilities. This system would work with the Print Rewind/Inspection System to create a label that contains all pharmaceutical information, but hidden for the purposes of blind testing. Once labels are approved – manually or automatically – in the first system, the labels will then be fed through the blind test labeling system.The Results: Improved Production Times by up to 95%
LabelPack Automation worked with representatives of the pharama company to come up with a solution that would solve two major problems. Before working with LabelPack, 80% of the company's prep work for clinical trials was done through outside contractors. Because so little was done in-house, the process took 3 – 4 months before clinical trials begin – a lifetime in the pharmaceutical world. Now, the system installed by LPA can complete the same job 3 – 4 days with 90% of the prep being done in-house.About The Client
The pharmaceutical company is an international leader in healthcare with headquarters in Bridgewater, New Jersey and is ranked the #3 top pharmaceutical company worldwide. The company develops and distributes solutions that is focused on what the patients need. Their strengths lie in, “diabetes solutions, human vaccines, innovative drugs, consumer healthcare, emerging markets, and animal health.”About Label Pack Automation
LabelPack Automation is an expert in the design and integration of custom labeling solutions in a variety of industries. Our solutions have been implemented in many different production lines to increase throughput, reduce costs, and drive results. LabelPack sales representatives and engineers are experienced in developing solutions for the pharmaceutical and food & beverage industries as well as providing a steady flow of supplies for mission-critical applications. Our labels are FDA, Pharma, and OSHA compliant. Contact LabelPack today and speak with a sales representative to learn how LabelPack Automation can help you achieve your business goals.Download this case study: